OVERVIEW
biology of tumor growth, invasion, and metastasis
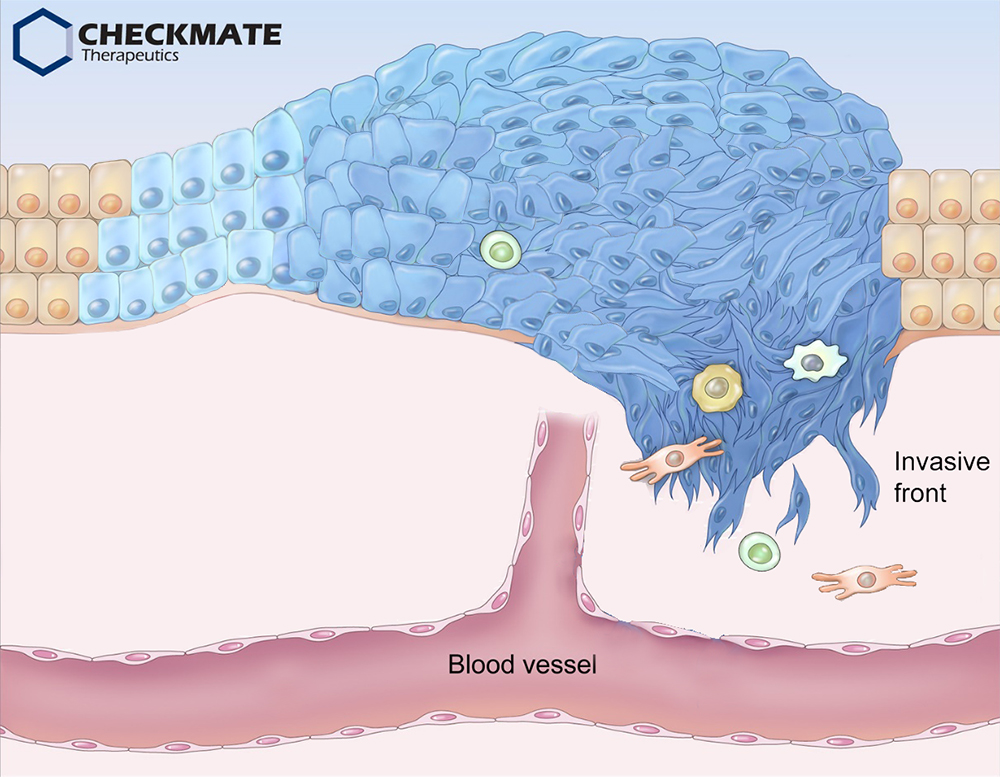
A tumor is not merely a simple mass of neoplastic cells; it also encompasses an extracellular matrix and a variety of other normal cells, including fibroblasts, endothelial cells, and immune cells 1. For tumor development, cancer cells recruit stromal cells and inflammatory immune cells into the tumor microenvironment. In turn, these recruited cells contribute to cancer progression via various mechanisms: Stromal and immune cells secrete cytokines and chemokines that directly promote cancer growth 2. Moreover, these cytokines induce the expression of programmed cell death ligands and other immune inhibitory ligands on the surface of tumor cells and antigen-presenting cells. They also elicit the accumulation of immune-suppressive cells, including M2-like tumor-associated macrophages (different from anti-tumor M1 macrophages), regulatory T cells, and myeloid derived suppressive cells, which help tumors escape immune surveillance via inhibition of cytotoxic T lymphocytes 1, 3.
On the invasive front, cancer cells undergo dynamic changes, called epithelial-mesenchymal transition (EMT) 4. EMT is a cellular process by which polarized epithelial cells gain highly motile and invasive mesenchymal properties that facilitate local invasion and dissemination to other organs, known as metastasis. In addition to promoting metastasis, EMT also confers cancer cells with resistance to chemo- and radio-therapy. Indeed, patients with tumors exhibiting elevated EMT features (found in around 20-30% of various solid tumors) face a poor prognosis and are refractory to currently available therapies, including chemotherapy (low proliferation rate), targeted therapy (lack of druggable mutations), and immune checkpoint blockade (low mutation burden) 5.
References
- 1) Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of Cancer: The Next Generation. Cell 144, 646-674.
- 2) Wang, M., et al. (2017). Role of tumor microenvironment in tumorigenesis. Journal of Cancer 8, 761-773.
- 3) Binnewies, M., et al. (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nature Medicine 24, 541-550.
- 4) Jung, H. Y., Fattet, L. and Yang, J. Molecular pathways: linking tumor microenvironment to epithelial-mesenchymal transition in metastasis. Clinical Cancer Research 21, 962-968.
- 5) Santamaria, P. G., et al. (2017). EMT: Present and future in clinical oncology. Molecular Oncology 11, 718-738.